There’s no denying the convenience of technology in our everyday lives. We can shop, sign mortgage documents, work, and learn a new language — all from the palm of our hand.
Tech is prominent in professional settings, too, helping businesses automate processes, streamline workflows, and improve communication and collaboration. It tightens data security and provides transparency — influencing how companies budget, sell, meet compliance standards, and boost productivity.
With all these benefits, it’s no surprise medical and pharmaceutical companies are using technology more in their clinical trials.
If you’ve ever participated in or conducted a clinical trial, there’s a good chance you’re familiar with case report forms, or CRFs. Either a clinical research coordinator or the trial participants themselves complete these documents — which include basic information like date of visit, blood pressure, height, and weight of each of the study’s participants.
CRFs should both identify all of the data elements required by the study’s sponsor and match the ones outlined in the protocol (e.g., participant name, gender, and ethnicity). A protocol is a written document that lays out the trial’s intent, safety measures, data and information security, and plan for measuring trial outcomes.
Put simply, CRFs help maintain and preserve the quality and integrity of the collected data and the clinical trial as a whole. And though it’s still common to use the traditional paper version of CRFs, electronic case report forms (eCRFs) are on the rise.
What is an eCRF, and why use one?
Clinical trial eCRFs are the electronic (or digital) versions of CRFs. (Note: Don’t confuse an eCRF with an electronic data capture (EDC) system. EDC systems are software that store clinical trial data like eCRFs and help clinical trial sites, sponsors, and contracted research organizations collect high-quality data more effectively and efficiently.)
Compared to paper-based CRFs, eCRFs are easier to analyze and generate reports from, especially if you have a large pool of participants. They also have other benefits:
- They’re more agile. With eCRFs, all the clinical trial data and documentation you need is in a central, digitized location, and all authorized personnel can access it quickly.
- They’re more efficient. Not only do you save money on paper by going digital, but clinical trial eCRFs also require less time and labor. Automating your eCRFs (instead of deciphering handwriting and manually transferring data) frees up time to work on other projects and tasks surrounding the clinical trial — like screening candidates, coordinating patients’ schedules, preparing board review documents, and providing patient care.
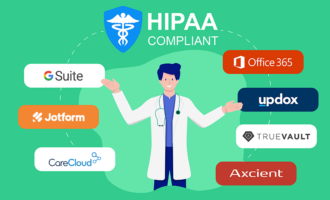
- They’re more secure. When it comes to highly sensitive data that needs to be protected — like Social Security and driver’s license numbers or financial information — medical records are at or near the top of the list. ECRFs help secure private information through password protection, storage that meets the requirements of Information Security Management System (ISO) 27001 and the Health Insurance Portability and Accountability Act (HIPAA), and an updated secure sockets layer (SSL) certificate. (An SSL certificate is an e-certificate that verifies a website’s identity and allows an encrypted connection between a web server and browser.)
- They’re more accurate. When completing paperwork manually, there’s always the possibility of human error. You might accidentally write your name in the space that requests your age or skip a field by accident. Clinical trial eCRFs prevent these mistakes by not allowing participants to move on to the next page or submit their paperwork unless they’ve entered all data in the required form fields, in the correct format.
This increases data quality and saves you from having to chase down your participants to make sure they fill out paper CRFs correctly. What’s more, since errors (like blank form fields) are detected automatically in most eCRFs (unlike in paper-based CRFs), your clinical trial results won’t be compromised.
- They’re more sustainable. If you’re conducting different clinical trials on similar topics, why reinvent the wheel? Instead, save yourself money and energy by reusing the same eCRF templates each time you start a new trial. Doing so will also standardize your eCRF process, ensuring everyone who works on each clinical trial poses the same types of questions to each participant in the same order (based on each trial’s protocol, of course).
How should you design clinical trial eCRFs?
Since eCRF data is integral to a successful clinical trial, the eCRF itself is as well. Too often, researchers simply copy paper-based CRFs verbatim in an electronic format without taking the time to use eCRF best practices and tweak questions, formatting, and structure to best fit a digital model. Well-designed eCRFs result in better data quality, reduced costs, and, ultimately, a shorter clinical trial journey from the discovery to post-market stages.
So, when it comes to designing high-quality clinical trial eCRFs — ones that your team can standardize and reuse — it’s important to keep these seven tips in mind.
1. Understand the eCRF’s intent
Before you even begin designing an eCRF, make sure you understand two critical factors about the associated clinical trial: its safety measures and intended results. Since those elements are key to the success of the clinical trial — and necessary for your participants to comprehend as they get started — they should be incorporated throughout the eCRF.
2. Receive input from the entire clinical trial team
From clinical research associates and site investigators to biostatisticians and research nurses, get input from as many people as you can as you build your eCRF. They may spot redundancies, unnecessary questions, or inconsistencies that might skew the results.
3. Create eCRF completion guidelines
Since the primary goal of eCRFs is to collect and record consistent, accurate data, it’s a good idea to create well-written, comprehensive eCRF completion guidelines as well. Proper completion guidelines can help combat confusion caused by lack of instruction, which is often cited as a problem within clinical trials. When you have eCRF completion guidelines in place, the clinical investigator will have clear, step-by-step instructions on how to complete the eCRF and conduct the trial — including diagnostic criteria, specific definitions of terms, and guidelines on how to handle inconsistencies and errors.
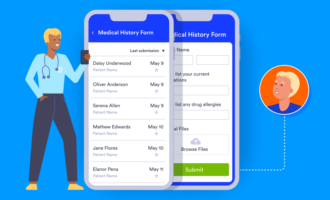
4. Ensure the eCRF is simple and consistent
Everything about your clinical trial eCRFs — including design, questions, and overall structure — should be user-friendly, consistent, and easily understandable for the clinical trial participants or research coordinators. Group similar questions together (participant demographics, vital signs, medical history, etc.) and consider making the response options predefined (not open-ended) for easier analysis. Use simple language, clean spacing, concise headers, and clear instructions throughout the entire eCRF.
5. Make eCRF fields mandatory
As previously mentioned, one of the biggest issues with traditional CRFs is that you run the risk of participants not completing a form field, whether or not they intended to skip it. Fortunately, you can require participants to answer all questions (or specific ones) with clinical trial eCRFs. You can also include a “data unavailable” or “N/A” option if you’re concerned about forcing someone to respond to a question they truly can’t answer accurately.
6. Run UAT
To ensure you’ve properly organized your eCRF and it will collect the correct data for the clinical trial, you should conduct User Acceptance Testing (UAT). The final phase of the software testing process, UAT — which your clinical trial team or paid, early testers can take care of — is your last chance to find mistakes or bugs before the eCRF goes live with your target participants.
7. Use the right software
Finally, designing dynamic, effective clinical trial eCRFs is easy with the right software — like online form builder Jotform. Using Jotform, you can
- Build eCRFs (including HIPAA-friendly online forms for added data security) from scratch or with one of Jotform’s premade templates
- Customize eCRFs with easy-to-use, drag-and-drop functionality, changing colors and fonts, inserting your logo, and adding and editing eCRF questions
- Safely collect, store, and share eCRFs, thanks to Jotform’s 256-bit SSL connection and form encryption
- Enable people to complete your eCRF from anywhere on any device (computer, tablet, or smartphone)
- Manage it all — eCRF clinical trial data, employees, participants, reports, and approval workflows — from one centralized platform
Jotform is comprehensive, intuitive, customizable, and fully equipped to handle all your clinical data management needs. Best of all, it’s completely code-free, allowing you to create powerful, well-designed eCRFs for a successful clinical trial — regardless of your programming experience.
When it comes to research, “bad data” (incomplete, inaccurate, or duplicate information) is worse than no data. This is especially true fdin the medical field, which is why digitized data collection has become so popular. It’s more agile, efficient, secure, and accurate than traditional, manually collected data, helping clinical trial participants and personnel feel more comfortable providing it and studying it.
So, whether you use Jotform or another solution to create and analyze your eCRFs, you now have a better understanding of what it takes to run a successful, streamlined clinical trial.




















































































Send Comment: