Informed consent forms ensure that people understand the risks involved in whatever they’re agreeing to. In some industries, these forms are mandatory and required by law.
There are two types of businesses where consent forms are most common:
- Healthcare organizations conducting clinical trials
- Companies selling high-risk activities that require a waiver — for example, adventure trips, zipline tours, or rollercoaster rides
While a consent form can’t prevent accidents from occurring or save people from themselves, such as this Royal Caribbean passenger who was banned for life after her failed selfie attempt, it can protect the company from being sued if the unexpected happens.
Here are some tips to help you put together a consent form to protect your company.
Pro Tip
Using an electronic informed consent form can help you grow your email list. An email list allows you to communicate with customers easily and can even be used for marketing purposes.
What elements should you include in your form?
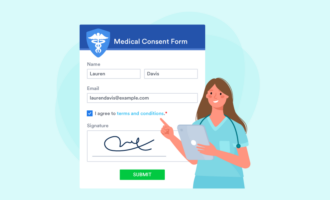
It might be tempting to cut some information from your consent form to keep it simple or save space. However, the form must include all of the elements you need from the person filling it out and all the information you need to relay to them.
For example, you should collect general information like the person’s name, mailing address, email address, date of birth, and medical history, where appropriate. Be sure to include a place for a signature as well.
You also need to provide certain information to the user. This should include the following when appropriate for your company:
Purpose of the study or research
In the medical field, patients must be informed of the reason for the study or trial. You should give them information about the treatments, including any experimental aspects.
Details of the procedure or event
This section should detail what the person can expect during the trial or event. It can also include the duration of the study and the circumstances that could end it.
Assurance of voluntary participation
The person signing the form must know that their participation is voluntary and that they can withdraw at any time.
List any risks
The risks shouldn’t be limited to the person signing the consent form. It’s important to note if the procedure or event could pose a threat or unexpected risk to others.
The person signing the form should understand potential risks or inconveniences as well as the potential positive outcomes.
Reimbursements or costs
The form needs to state any reimbursements that will be paid to the participant or what the financial cost to the person is. It should also state the compensation or treatment available should something go wrong.
Confidentiality statement
The form must include any regulatory authorities, auditors, or monitors that will be granted access to the person’s information, if applicable.
Additional legal information
You might need to include additional mandatory information in your forms, depending on your industry and the purpose of the form. For example, the FDA regulates what must be included on an informed consent form for clinical trials.
Pro Tip
Use Jotform Sign Mobile to invite patients by link or email, capture in-person consent during visits, scan paper to e-sign, and track status in Sign Inbox.
Best practices for building informed consent forms
Here are some factors to keep in mind when creating your digital informed consent form.
Create a user-friendly layout
Paper forms are generally boring and restricted to a certain number of pages. Digital forms don’t have the same restrictions, so you can create a more user-friendly experience. For example, you can make the form easier to navigate by breaking the information into sections or different pages.
Automate responses
Some fields in a form can be filled in automatically to save the user time — for example, the date and time that the form is signed. If the person already has an account on your website, information like name and address can be automatically populated in the form.
Use conditional logic
Your form may hide or show questions based on previous responses. For example, if the person filling out the form responds yes to a question that asks if they have children, the form could then show a question that asks for the age of the children. If the answer to the question about having children was no, then the form would skip the question about the age of the children.
Use contextual support (i.e., help text)
Providing help text within the form can guide the user through the questions. In a paper form, you don’t have the space to elaborate on complex questions. In digital format, you can include explanations and directions that pop up when the person hovers over or taps on specific text.
Keep it concise
Short and sweet is the best way to go. Of course, you need to include all of the necessary information and questions, but only keep what you really need. Don’t throw in extra questions just to have them. Make sure every question serves a purpose.
What are the 4 principles of informed consent?
- Patient must be competent enough to be able to make a free choice. Patient must not be mentally handicapped
- Consent must be granted willingly and freely
- Content of the consent must be understood completely
- Patient must be able to rationally manipulate the given information
The most important thing to keep in mind when creating your electronic informed consent form is the end user.
Make sure the content is clear, contains all of the mandatory elements, and is simple to navigate. Don’t be afraid to revisit your form from time to time, and look for ways you can improve it.









Send Comment: